A major UK trial looking at whether Covid vaccines can be mixed with different types of jabs used for first and second doses is being expanded.
Combining vaccines might give broader, longer-lasting immunity against the virus and new variants of it, and offer more flexibility to vaccine rollout.
Adults over 50 who have had a first dose of Pfizer or AstraZeneca can apply to take part in the Com-Cov study.
Their second dose could be the same again, or a shot of Moderna or Novavax.
Chief investigator on the trial, Professor Matthew Snape, from the Oxford Vaccine Group, said he hoped to recruit 1,050 volunteers who have already received one dose on the NHS in the past 8-12 weeks.
More than 800 people are already taking part in the research and have received two doses of either Pfizer, AstraZeneca or a mix.
Results of this first stage are expected next month, and the expanded trial should have some reportable findings by June or July – although the study will run for a year.
Health experts generally agree that the mixing and matching of the vaccines should be safe. The trial will check for any side-effects or unwanted reactions.
Participants will have blood taken to check how well the vaccines trigger an immune response – in the form of antibodies and T cells – to combat Covid.
Prof Snape said: “If we can show that these mixed schedules generate an immune response that is as good as the standard schedules, and without a significant increase in the vaccine reactions, this will potentially allow more people to complete their Covid-19 immunisation course more rapidly.
“What I’m hoping is that we won’t rule out any combinations.
“That’s how we need to look at it: are there any combinations we shouldn’t be giving, because they don’t generate a good immune response? And I’m hoping that won’t be the case.”
He said the option to mix vaccines “will give us lots of flexibility, not just in the UK, not just in Europe – where we’re looking at restricting use of some vaccines for some age groups – but across the world, where we have, perhaps, a little bit more intermittent supply of vaccines.
“Let’s hope that we can actually use this to get two doses of vaccine to as many people as possible.”
Meanwhile, in other developments:
- People aged 45 and over are being invited to make appointments for their Covid vaccinations in England, while Scotland is set to follow suit this week. In Northern Ireland and Wales, people aged over 40 are eligible.
- The US, South Africa and the European Union have temporarily halted the rollout of the Johnson and Johnson single-dose vaccine after reports of rare cases of blood clotting – similar to those seen with the AstraZeneca jab.
- Scotland is preparing to lift restrictions on travelling within the nation from Friday, as well as allowing larger outdoor gatherings of up to six people, each from different households.
- An outbreak of the South Africa variant in south London, which has prompted surge testing in two boroughs, is thought to have begun with an individual who travelled from Africa in February, official documents seen by the BBC suggest.

- VACCINE: When will I get the jab?
- SOCIAL DISTANCING: How can I meet my friend safely?
- LOOK-UP TOOL: How many cases in your area?
- LOCKDOWN RULES: What are they and when will they end?

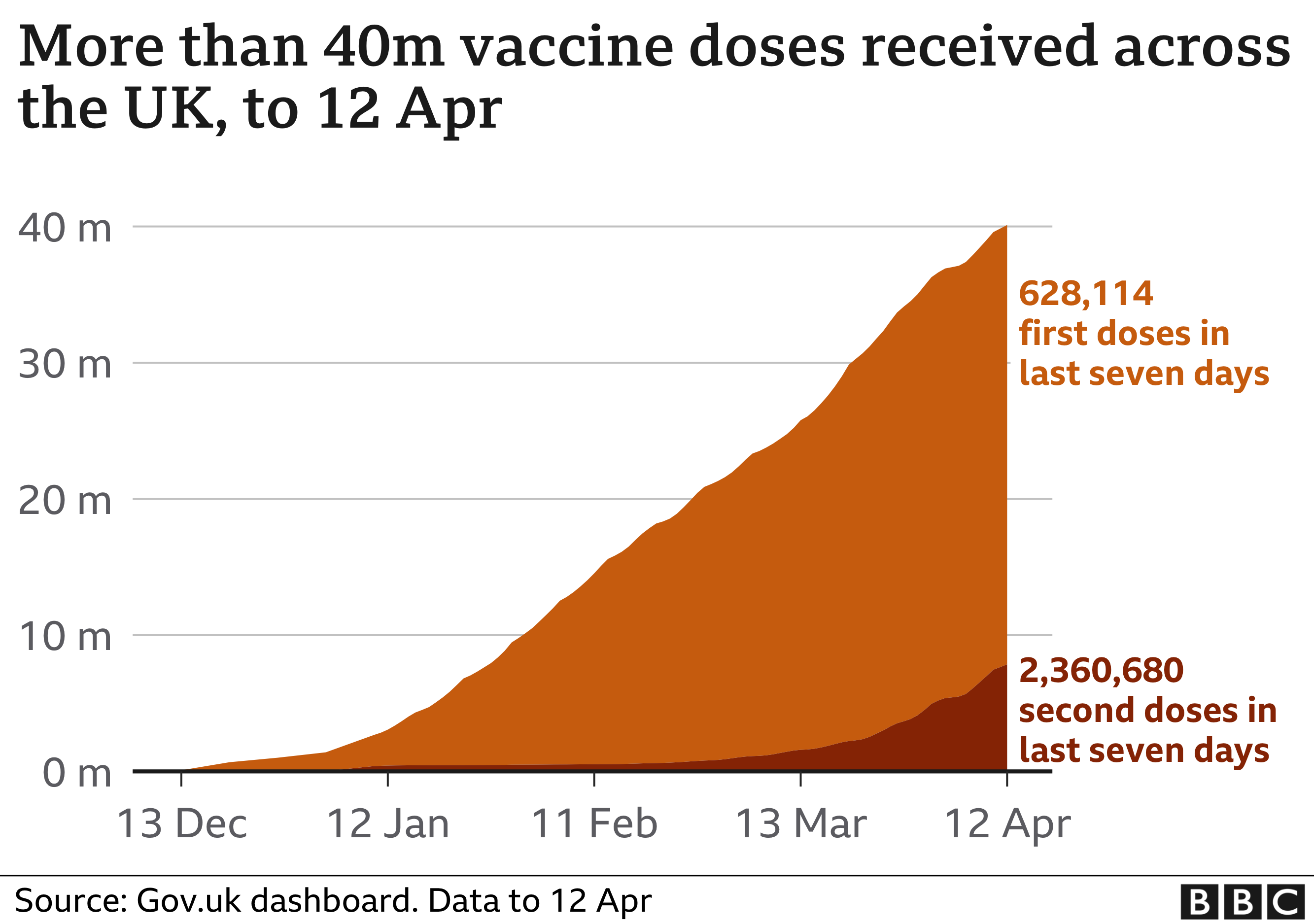
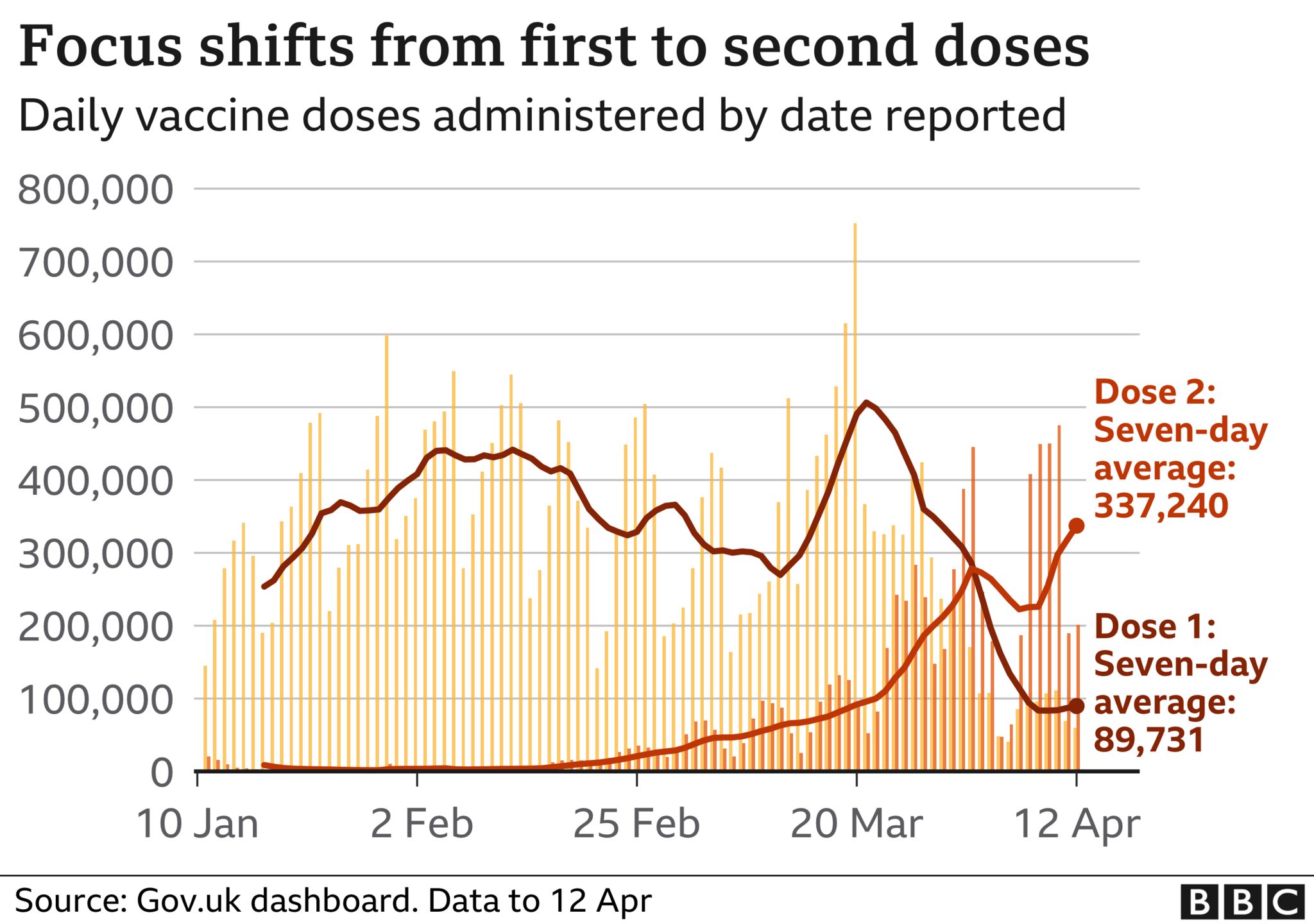
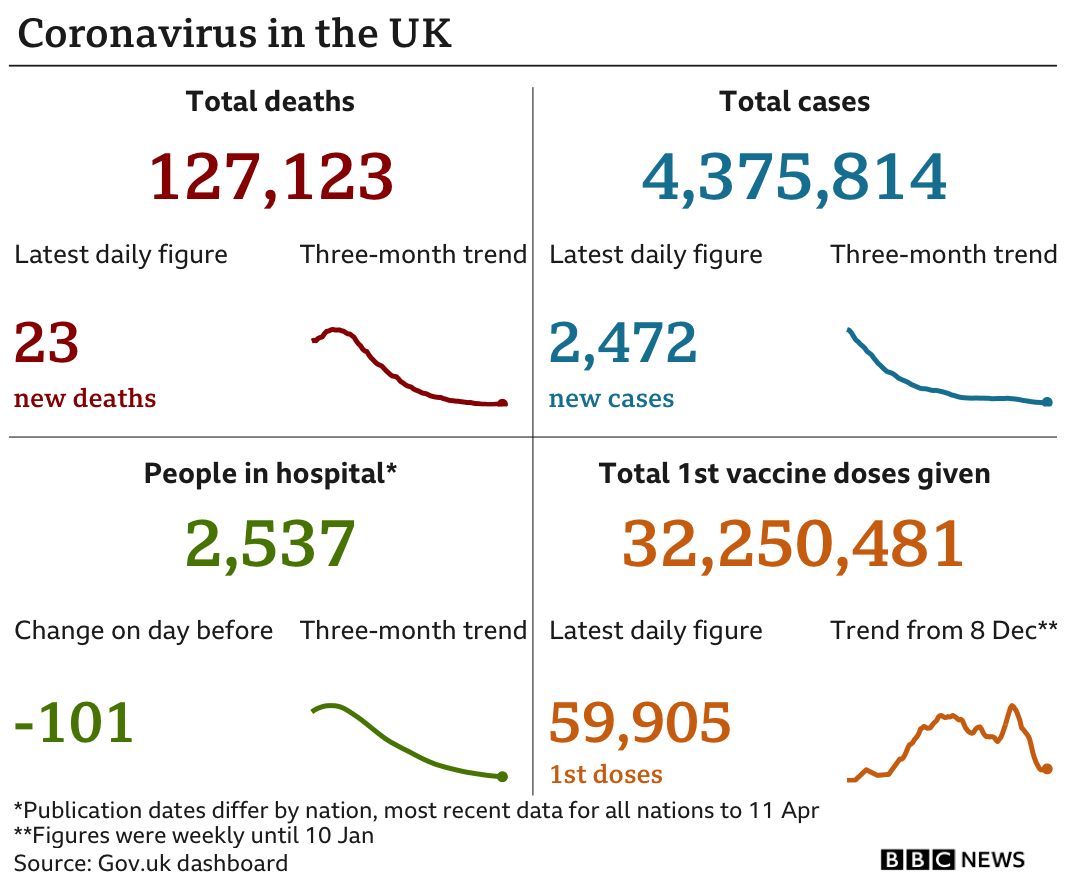
So far more than 32 million people in the UK have received only the first dose of a coronavirus vaccine, while 7.8 million people have been given both doses.
Outside of the trial, people should still receive the same type of Covid-19 vaccine for their first and second doses, although they can be given different brands if the same vaccine is not available.
The Moderna vaccine is already approved for use in the UK and works in a similar way to the Pfizer/BioNTech one, using a small amount of genetic code from coronavirus to teach the body how to fight off infection.
The Oxford/AstraZeneca vaccine is slightly different and uses a harmless, modified virus to carry instructions on how to beat Covid.
The Novavax jab has not been approved yet in the UK, but is expected to be soon, since trials show it is safe and effective.
It uses proteins from coronavirus that can train the immune system without causing infection.
The UK is not the only country considering using mixed dosing.
Head of China’s Center for Disease Control and Prevention, George Gao, recently said China should consider it to boost vaccine effectiveness.
Russia’s Sputnik V vaccine involves using two slightly different doses to give immunity.
Prof Jeremy Brown, a member of the UK’s Joint Committee of Vaccination and Immunisation, which advises on which vaccines to give to the population, said in coming years, people will eventually “have to” have a mix of Covid-19 jabs.
He told the BBC: “It’s practically going to have to be that way because, once you’ve completed a course of, say, the Moderna or Pfizer or the AstraZeneca, with two doses – in the future, it’s going to be quite difficult to guarantee you get the same type of vaccine again.”
The trial of mixed vaccines is funded by the vaccine taskforce and supported by the National Institute for Health Research. It is being run from nine different sites across England:
- St George’s University Hospitals NHS Foundation Trust
- University Hospitals Birmingham NHS Foundation Trust
- The University of Nottingham Health Service
- Liverpool School of Tropical Medicine
- University College London Hospitals NHS Foundation Trust
- Hull University Teaching Hospitals NHS Trust
- The Newcastle Upon Tyne Hospitals NHS Foundation Trust
- Guy’s and St Thomas’ NHS Foundation Trust
- Sheffield Teaching Hospitals NHS Foundation Trust

- A DIFFERENT TAKE ON THE BIGGEST FOOTBALL STORIES: From Man U to Watford with The Squad
- “AT ONE POINT I THOUGHT MY LIFE WAS OVER”: The ‘friendly fire’ in my brain